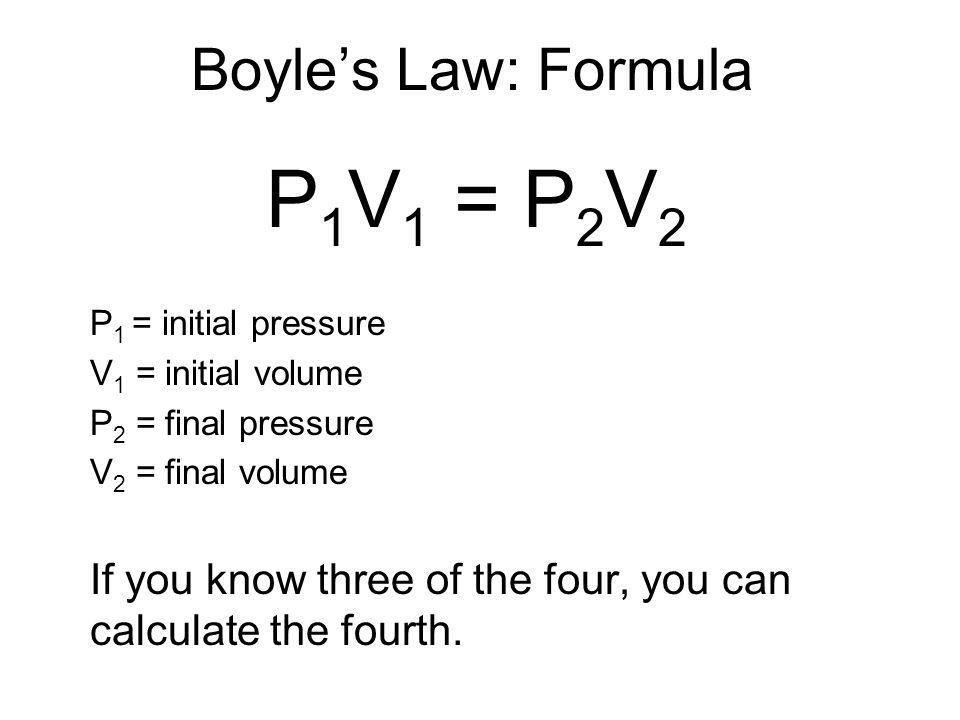
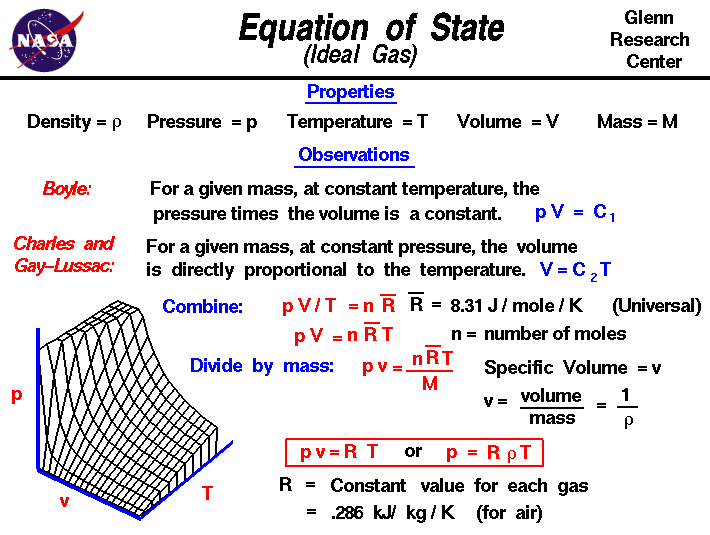
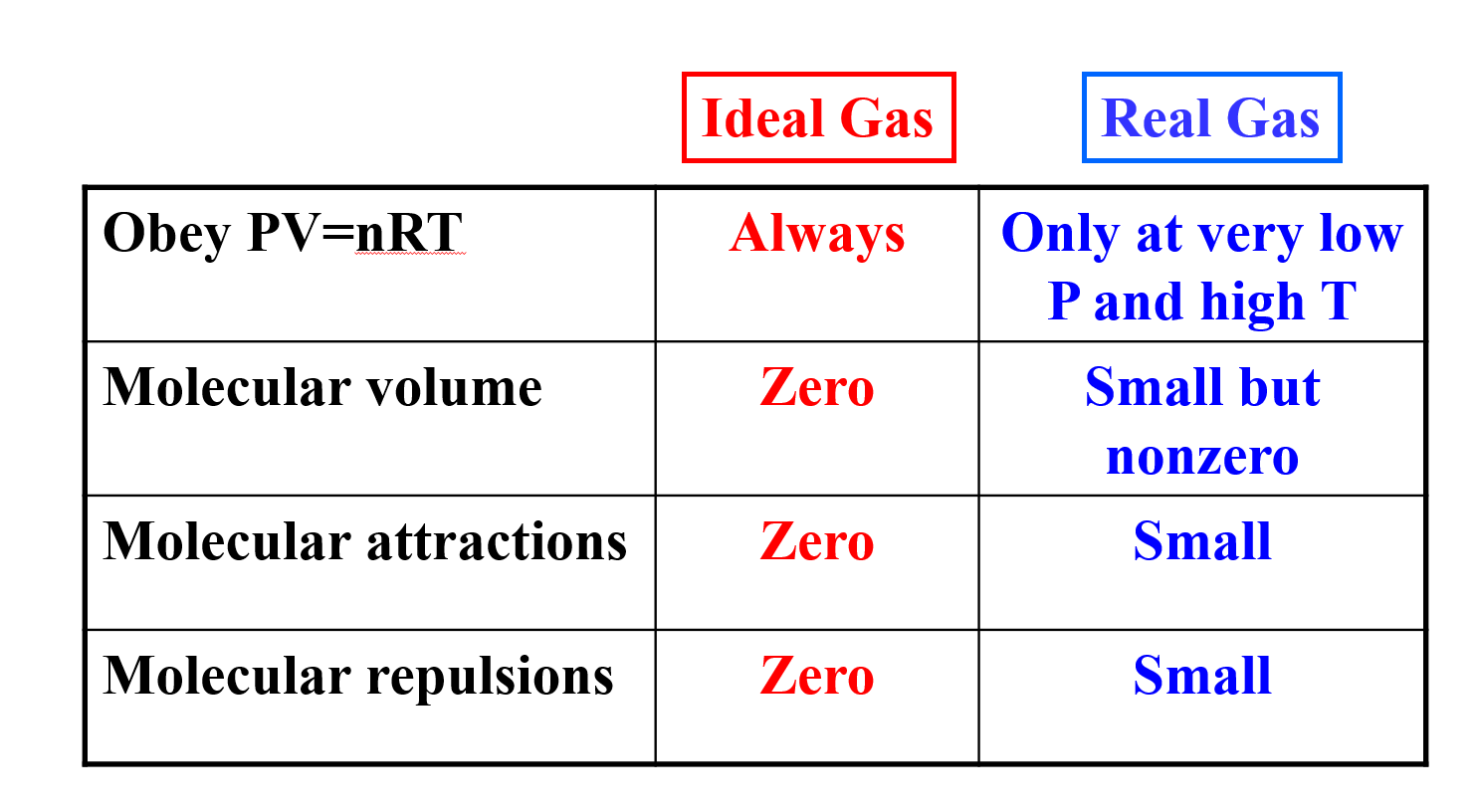
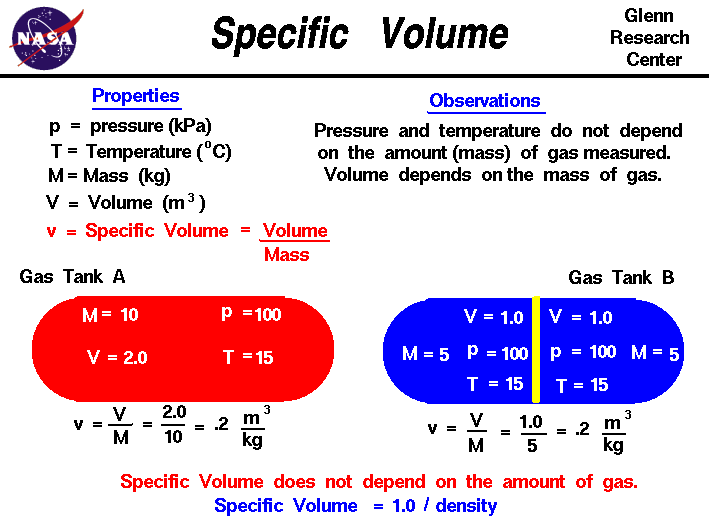
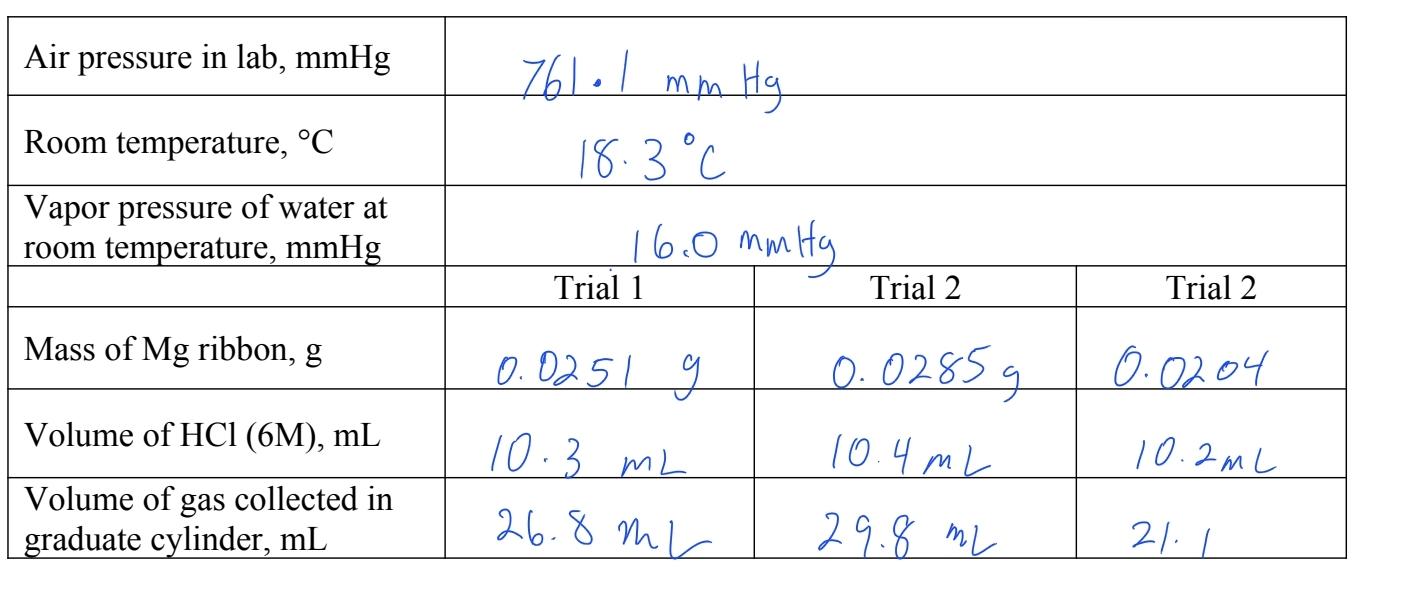
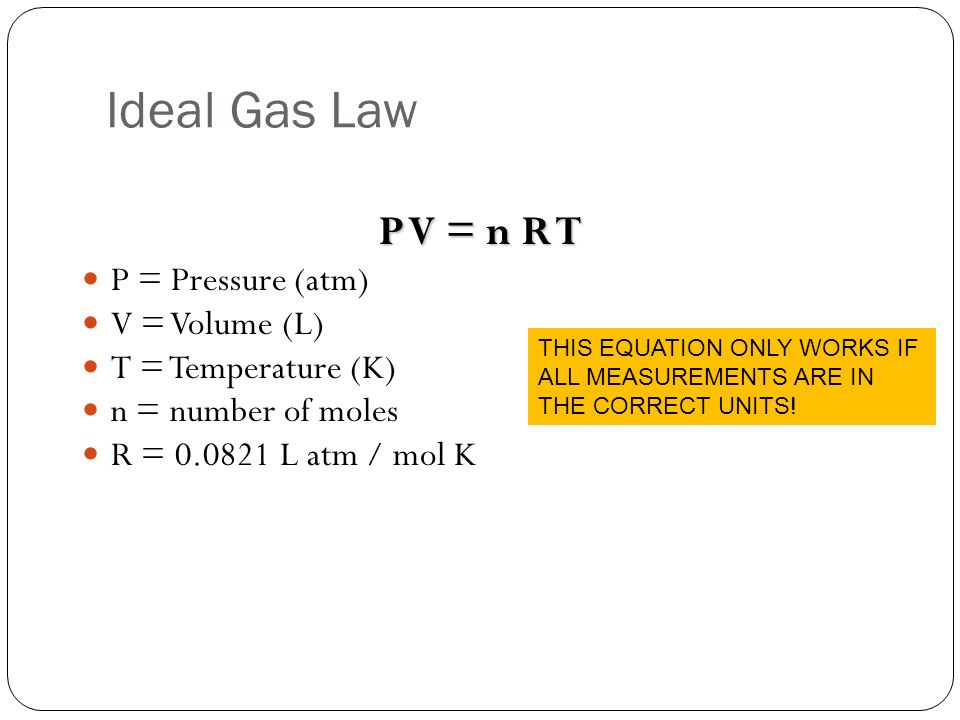
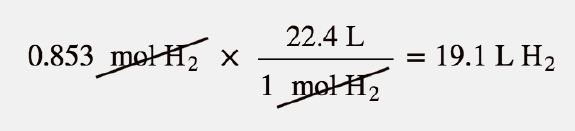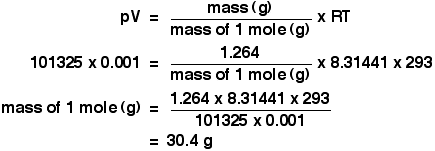Welcome to Chem Zipper.com......: Find the Volume of gas evolved by passing 0.965 Amp current for 1 hour through an aqueous solution CH3COONa at 250c and 1 atm?

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

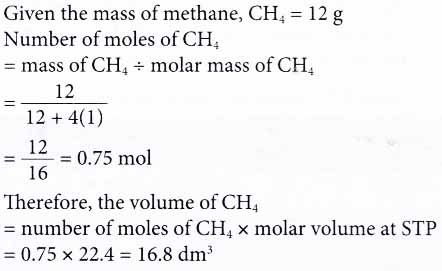
SOLVED:Use molar volume to calculate each of the following at STP: a. the volume, in liters, of 6.40 \mathrm{g} of \mathrm{O}_{2} gas b. the number of grams of \mathrm{H}_{2} in 1620 \mathrm{mL}
![High School:Stoichiometry] Calculating volume of gas. I got 400 but even my teacher only got 480. The answer is C. : r/chemistryhomework High School:Stoichiometry] Calculating volume of gas. I got 400 but even my teacher only got 480. The answer is C. : r/chemistryhomework](https://i.redd.it/j69a24fc57y31.jpg)
High School:Stoichiometry] Calculating volume of gas. I got 400 but even my teacher only got 480. The answer is C. : r/chemistryhomework

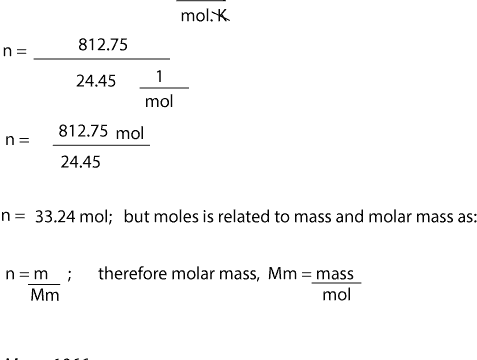
SOLVED:Calculate the volume of each gas sample at STP. (a) 48.9 \mathrm{g} \mathrm{He} (b) 45.2 \mathrm{g} \mathrm{Xe} (c) 48.2 \mathrm{mg} \mathrm{Cl}_{2} (d) 3.83 \mathrm{kg} \mathrm{SO}_{2}