Industry's Giant Leap Into Cellular Therapy: Catalyzing Chimeric Antigen Receptor T Cell (CAR-T) Immunotherapy | SpringerLink
Novartis fills manufacturing gap for CAR-T therapy Kymriah with first Asian production facility | Fierce Pharma
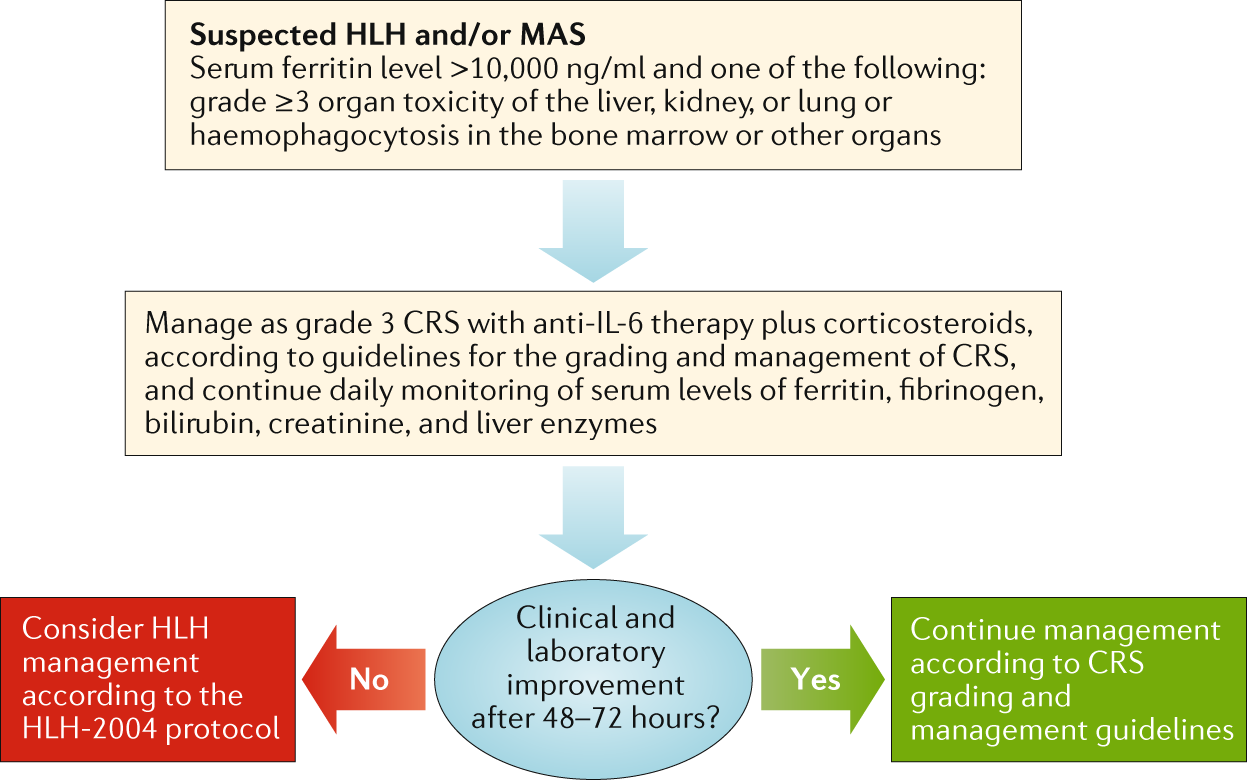
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology

How to cover Novartis' $475K CAR-T drug Kymriah? A 'new payment model' is the only way, Express Scripts says | Fierce Pharma

PDF) Chimeric Antigen Receptor-T Cell Therapy: Practical Considerations for Implementation in Europe
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

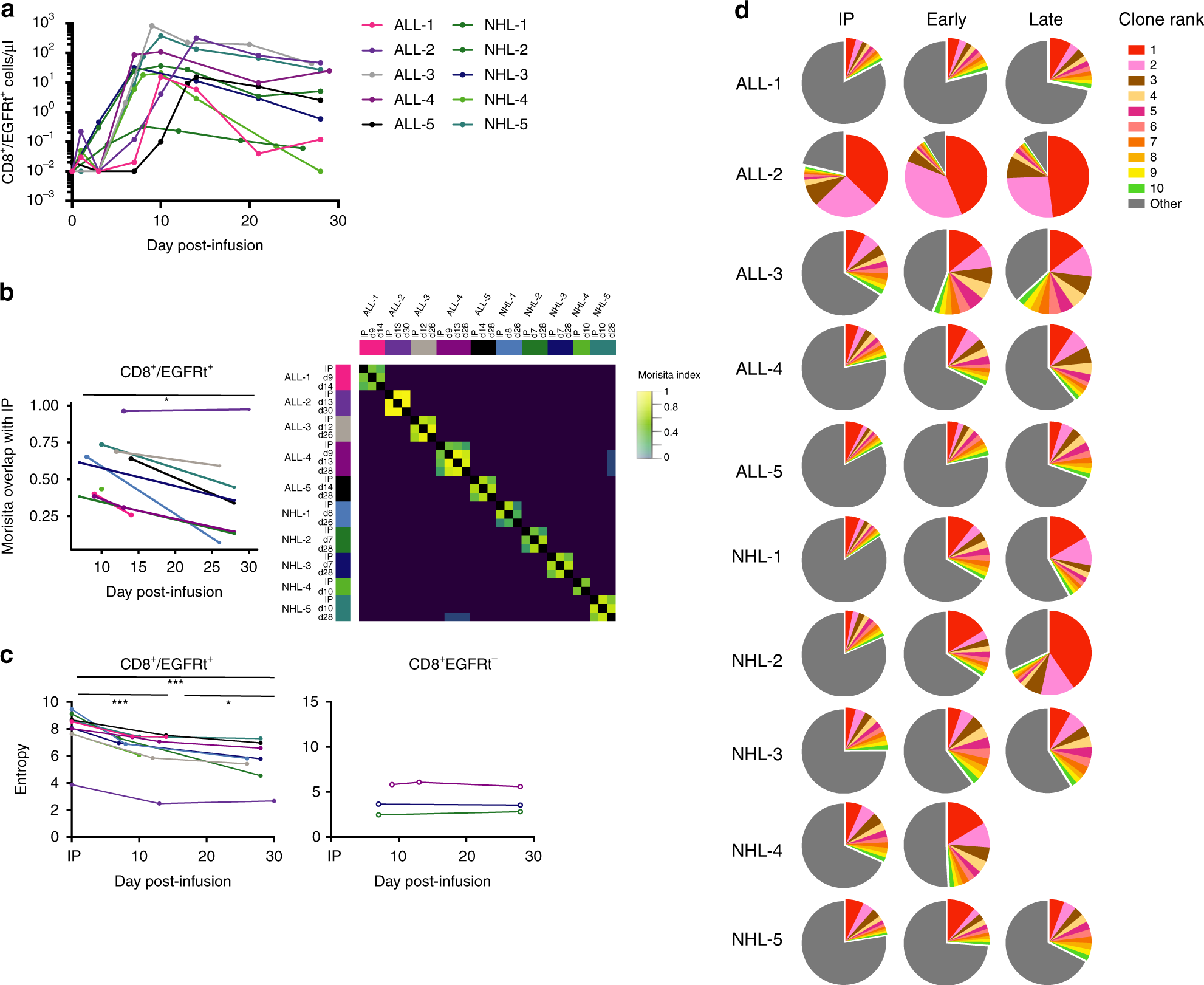
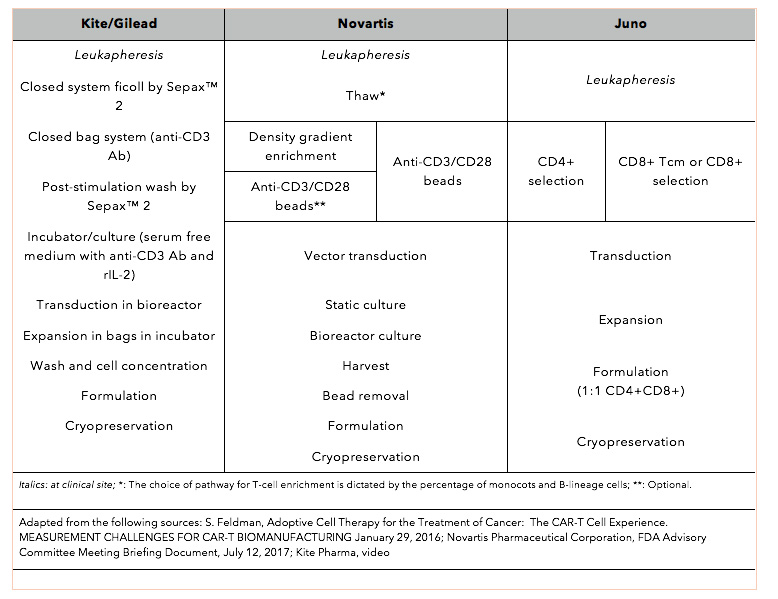
Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19 - Cytotherapy

FDA Advisory Committee Recommends Approval of CAR-T Cell Therapy and Two New Biosimilars | Biosimilars Law Bulletin

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

CAR-T Cell Product Development Guidance Covers Previous Recipients, 'Bridging Therapy' :: Pink Sheet
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar